Chromate conversion coating is one of many types of conversion coating. Chromate Conversion is also known as Chromatizing or Chromating
Conversion Coatings:
Metal and alloy coating is one of the most common means of protection against corrosion. Coatings also provide other properties, such as mechanical, physical and also esthetic.
Commonly used coatings are inorganic non-metal coatings. These can be divided to so-called contact and conversion coating group. Contact coatings are for example enamel, cement and ceramic coatings. These coatings are applicable on any metal or alloy, regardless of the manufacture procedure, their composition and inherent thickness; these coatings are non-selective.
On the other hand, conversion coatings are selective. The reason for this is the fact that the coating is formed by reaction between ions in the deposition bath and metal cations dispersed to the bath during the initial corrosion reaction between the bath and coated metal.
Chromate Conversion Coating
After steel phosphating or aluminium anodizing (eloxing), chromate process is the most common conversion surface treatment. Chromate coatings serve as additional corrosion protection layer predominantly used on electrodeposited zinc and cadmium coatings. These coatings are also used for protection and for aesthetic reasons as a final surface layer on zinc castings, moldings and semi-finished magnesium products and its alloys as well as on copper and its alloys. They are often used as interlayer providing adhesive surface for both conventional and powder paints. It can be alternatively used as absorbent layer for lubricant, however phosphate layers produce better results.
Chromate Conversion Coating are formed on metal surfaces as a result of the chemical attack that occurs when a metal is immersed in or sprayed with an aqueous solution of chromic acid, chromium salts such as sodium or potassium chromate or dichromate, hydrofluoric acid or hydrofluoric acid salts, phosphoric acid, or other mineral acids. The chemical attack facilitates the dissolution of some surface metal and the formation of a protective film containing complex chromium compounds.
Metals that can be Chromatized
A variety of metals and electrodeposited metal coatings can be conversion coated…
- zinc,
- cadmium,
- magnesium,
- aluminum
- copper, silver, titanium, magnesium, and tin alloys
Chromatizing Mechanism
Chromate conversion treatments actually take advantage of this high surface activity. Through use of a strong oxidizing agent such as chromic acid, CrO3, a redox reaction occurs at acidic pH (pH — 2) where hexavalent chromium, either in the form of Cr2O7 or HCrO4, is reduced to trivalent chromium while aluminum is oxidized to trivalent aluminum.

another reduction reaction besides that involving chromic acid can occur. This reaction involves the reduction of either water, hydronium ion, or dissolved oxygen to form hydroxyl ions at the metal surface.

This surface-localized increase in pH results in the precipitation of an amorphous mixture of hydrated aluminum plus chromium oxides.
The high corrosion resistance offered by chromate films is attributed to the presence of both hexavalent and trivalent chromium in the coating. Analyses of coatings by wet chemical methods and with surface-sensitive techniques have shown that both Cr(VI) and Cr(III) are present in the films. The trivalent chromium is believed to be present as an insoluble hydrated oxide, whereas the hexavalent chromium imparts a “self-healing” character to the film during oxidative (corrosive) attack by species such as chloride ion. The hexavalent chromium is reduced during corrosion to form an insoluble trivalent chromium species that terminates the oxidative attack.
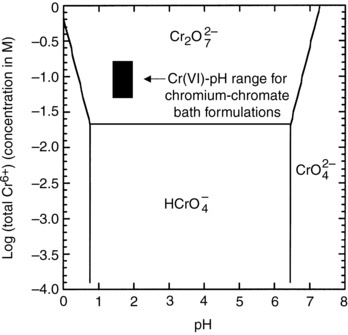
Formation of chromium chromate coatings is normally carried out at pH values where dichromate is the predominant Cr(VI) species.
Bath condition:
- Solution concentrations= 50 mM Cr(VI)
- pH = 1.2 to 3.0
Chromate Conversion Coating solution is applied to surface by
immersion
spraying
Sequence for Chromate Conversion Coating – Chromating

Factors affecting Coating thickness & its corrosion resistance in Chromate Conversion Coating
1) Substrate Metal
The ease with which coatings on aluminum can be produced, and the degree of protection afforded by them, can vary significantly with the alloying constituents and/or the heat treatment of the part being processed
- Alluminum Alloys
- Low alloying constituent metals that are not heat treated are easiest to chromate and provide the maximum resistance to corrosion.
- wrought aluminum, which is high in alloying elements (especially silicon, copper, or zinc) or which has undergone severe heat treatment, is more difficult to coat uniformly
- Magnesium Alloys
- As in the case of aluminum, the alloying element content and the type of heat treatment affect the chromating of magnesium
- Zinc Alloys
- Chromate conversion coatings on zinc electroplate are affected by impurities codeposited with the zinc. For example, dissolved cadmium, copper, and lead in zinc plating solutions can ultimately cause dark chromated films.
2) Effect of pH
For any given metal/chromate solution system, there will exist a pH at which the rate of coating formation is at a maximum. As the pH’is lowered from this point, the reaction products increasingly become more soluble, tending to remain in solution rather than deposit as a coating on the metal surface.
3) Activators
Chromate films normally will not form without the presence of certain anions in regulated amounts. They are commonly referred to as “activators’ and include acetate, formate, sulfate, chloride, fluoride, nitrate, phosphate, and sulfamate ions.
Example. Fluoride is particularly effective in promoting film. F dissolves the oxide film initially present on the aluminum surface and activates the surface for the deposition reactions to proceed.
5) Operating Conditions
The following factors also govern film formation. Once established for a given operation, these parameters should be held constant.
- Treatment Time
- Solution Temperature.
- Solution Agitation
6) Rinsing
Once a chromate film has been formed satisfactorily, the surface should be rinsed as soon as possible. Transfer times from the chromating stage to the rinsing stage should be short in order to minimize the continuing reaction that takes place on the part.
Film Characteristics and Properties of Chromate conversion coating (Chromatizing)
It is primarily used due to its film characteristics and specific applications
- Film Physical Characteristics
- Most chromate films are soft and gelatinous when freshly formed. Once dried, they slowly harden or “set” with age and become hydrophobic, less soluble, and more abrasion resistant.
- Variety of colors normally are obtained on chromating, and are due mainly to interference colors of the thinner films and to the presence of chromium compounds in the film.
- Corrosion Inhibitor
- Protection is due both to the corrosion-inhibiting effect of hexavalent chromium contained in the film and to the physical barrier presented by the film itself. Even scratched or abraded films retain their protection because of self healing of hexavalent chromium
- The degree of protection normally is proportional to film thickness.
- Bonding of organic finishes
- The bonding of primers, paints, lacquer, and organic finishes to chromate conversion coatings is excellent. In addition to promoting good initial adhesion, their protective nature prevents subsequent loss of adhesion that is due to underfilm corrosion.
- Decorative finish due to dying color capability
- Retain electrical conductivity
- The contact resistance of articles that have been protected with a chromate conversion coating is generally much lower than that of an unprotected article that has developed corroded or oxidized surfaces. Less resistance means higher conductivity.
| Metal | Corrosion resistance | Paint base | Chemical polish | Metal coloring | Remarks |
| Aluminum | X | X | … | X | Economical replacement for anodizing if abrasion resistance is not required. Used to “touch-up” damaged areas on anodized surfaces |
| Cadmium | X | X | X | X | |
| Copper | X | X | X | X | |
| Magnesium | X | X | … | … | |
| Silver | X | … | … | … | |
| Zinc | X | X | X | X |
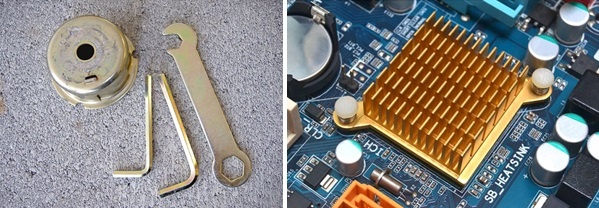
Applications Parts of Chromate Conversion Coating
- Tools, Small Components of various machines, bolts, heat sinks, bobbins in sewing machine, hand tool wrenches, pins, rings etc.
You can enhance your knowledge about Chromate conversion coating from references below
References
1. “Chromate Conversion Coatings”, ASM Handbook, Volume 5: Surface Engineering
2. Fred W. Eppensteiner, “Chromate Conversion Coatings”, Metal Finishing, Elsevier 2001
3. P. Pokorny, P. Tej, “Chromate Conversion Coatings And Their Current Application”, Metalurgija, April 2016
