Boyle’s Law is a fundamental principle in physics that describes the relationship between the volume and pressure of a gas at a constant temperature. It was named after the Irish scientist Robert Boyle, who discovered the law in 1662. This article will explain Boyle’s Law, its equation, and provide some examples to help readers understand its practical applications.
The Basics of Boyle’s Law
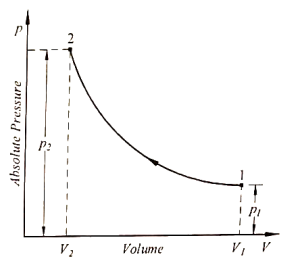
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. In simpler terms, if the volume of a gas decreases, the pressure will increase, and if the volume increases, the pressure will decrease, as long as the temperature remains constant.
This relationship can be seen in the behavior of gases when they are compressed or expanded. For example, when air is pumped into a balloon, the pressure inside the balloon increases, causing it to expand. Similarly, when air is released from the balloon, the pressure decreases, causing it to deflate.
It’s important to note that Boyle’s Law only applies to ideal gases, which are theoretical gases that have no intermolecular forces and occupy no volume. However, real gases can often be approximated as ideal gases under certain conditions.
Boyle’s Law Equation
Boyle’s Law can be expressed mathematically using the following formula:
P1V1 = P2V2
Where:
- P1 is the initial pressure of the gas
- V1 is the initial volume of the gas
- P2 is the final pressure of the gas
- V2 is the final volume of the gas
This equation is also known as the Boyle-Mariotte Law, named after the French scientist Edme Mariotte, who discovered it independently of Boyle.
To use this equation, the units of pressure and volume must be consistent. In SI units, pressure is measured in Pascals (Pa) and volume in cubic meters (m3). In practice, other units such as atmospheres (atm) or liters (L) may be used.
Boyle’s Law Examples
To better understand the practical applications of Boyle’s Law, let’s consider some examples.
Balloon Experiment
Imagine a balloon filled with a fixed amount of gas at a certain temperature. If the balloon is squeezed or compressed, the volume of the gas inside the balloon will decrease, and the pressure will increase according to Boyle’s Law. Conversely, if the balloon is allowed to expand, the volume of the gas will increase, and the pressure will decrease.
SCUBA Diving
SCUBA divers breathe compressed air from a tank while diving underwater. The pressure in the tank is much higher than the pressure at the surface, so the air is compressed to fit more of it into the tank. As the diver descends deeper into the water, the pressure around them increases, causing the compressed air in their tank to decrease in volume. To maintain a constant supply of air, the regulator attached to the tank releases air into the diver’s lungs at a pressure equal to the ambient pressure around them.
Tire Pressure
Boyle’s Law is also applied in tire pressure maintenance. When a tire is inflated with air, the volume of the air inside the tire is increased, and the pressure is higher than the outside air pressure. As the tire rolls on the road, the pressure decreases, and the volume of the air inside the tire increases. Therefore, to maintain a safe and efficient driving experience, it is important to regularly check and adjust the tire pressure according to the manufacturer’s specifications.
Practical Applications of Boyle’s Law
Boyle’s Law has many practical applications in various industries, including:
Medical Equipment
Boyle’s Law is used in respiratory therapy equipment such as ventilators and anesthesia machines. These machines regulate the pressure and volume of air or oxygen that is delivered to the patient’s lungs to maintain proper breathing.
Climate Control Systems
Boyle’s Law is used in refrigeration and air conditioning systems. The cooling process involves compressing a gas, which increases its temperature and pressure. Then, the compressed gas is allowed to expand, which causes it to cool down. This cycle is repeated to maintain a comfortable indoor temperature.
Limitations of Boyle’s Law
Boyle’s Law has certain limitations. It only applies to ideal gases at constant temperature, which is rarely the case in real-world scenarios. Additionally, other factors such as intermolecular forces and gas composition can affect the behavior of gases.
Conclusion
Boyle’s Law is an important principle in physics that describes the relationship between gas pressure and volume. It has many practical applications in various industries, including medical equipment and climate control systems. Understanding this law can help us better understand the behavior of gases and make informed decisions in our daily lives.
Boyle’s Law Calculator
Learn More…
FAQs
- Who discovered Boyle’s Law?
- Boyle’s Law was discovered by the Irish scientist Robert Boyle in 1662.
- What is the formula for Boyle’s Law?
- The formula for Boyle’s Law is P1V1 = P2V2.
- What are some practical applications of Boyle’s Law?
- Boyle’s Law has many practical applications in various industries, including medical equipment, climate control systems, and tire pressure maintenance.
- What are the limitations of Boyle’s Law?
- Boyle’s Law only applies to ideal gases at constant temperature and doesn’t take into account other factors that can affect gas behavior.
- Why is it important to maintain proper tire pressure?
- Maintaining proper tire pressure is important for safety and fuel efficiency. An underinflated tire can cause poor handling and increased fuel consumption, while an overinflated tire can increase the risk of a blowout.
- What are some real-life examples of Boyle’s Law?
- A syringe needle gets thinner as you push the plunger down because the volume of the gas in the syringe decreases, increasing the pressure according to Boyle’s Law.
- A scuba diver must equalize the pressure in their ears as they descend in the water to avoid damage to their eardrums, which is also governed by Boyle’s Law.
- Can Boyle’s Law be applied to liquids or solids?
- Boyle’s Law only applies to gases, as their volume is easily changeable with pressure.
- How does temperature affect Boyle’s Law?
- Boyle’s Law assumes that temperature remains constant. If the temperature changes, then the gas constant (R) also changes, altering the relationship between pressure and volume.
- How is Boyle’s Law related to the ideal gas law?
- Boyle’s Law is one of the four gas laws that make up the ideal gas law, which describes the behavior of ideal gases at different temperatures and pressures.
- What is the difference between Boyle’s Law and Charles’ Law?
- Charles’ Law describes the relationship between gas volume and temperature at constant pressure, while Boyle’s Law describes the relationship between gas pressure and volume at constant temperature.