This article contains CVD Process introduction, principle, variants, variables, advantages, applications
Introduction to CVD Process
- Chemical vapor deposition (CVD) is a family of processes whereby a solid material is deposited from a vapor by a chemical reaction occurring on or in the vicinity of a substrate surface.
- The resulting solid material is in the form of a thin films(Å to μm) , powder, or single crystal.
- By changing variables, thin films with variety of physical, tribological, and chemical properties can be grown.
- CVD technique has excellent throwing power à coatings of uniform thickness and properties with a low porosity even on substrates of complicated shape.
- Another important feature is the capability of selective deposition, on patterned substrates.
Principle & Process of CVD
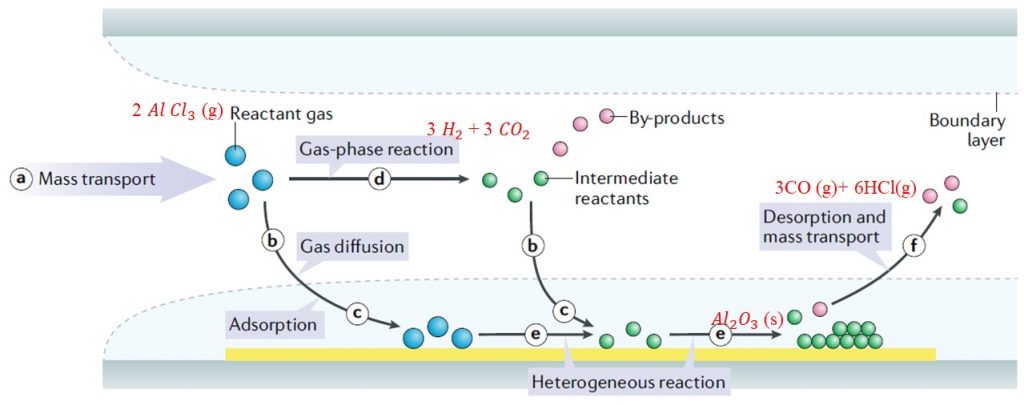
Gaseous reactants(precursor) (g)→Solid material (s) + Gaseous by-products (g)
a. Reactant gases (blue circles) are transported into the reactor by forced convection.
Then, there are two possible routes for the reactant gases:
route b. Diffusing through the boundary layer
route d. Forming intermediate reactants (green circles) and by-products (red circles) via the gas-phase reaction (step d)
c. Adsorbing onto the substrate
e. Surface diffusion and heterogeneous reactions take place on the surface of substrate before the formation of thin films or coatings. including chemical decomposition, surface migration to attachment sites (such as atomic-level ledges and kinks).
f. By-products and unreacted species are desorbed from the surface boundary layer and forced out of the reactor as exhausts
Principle Reactions in CVD Process
Here are some of the reactions, apart from these many reactions take place. End goal of reaction is to have precursor gas→ solid film + gaseous by-products
| Pyrolysis Thermal decomposition of gaseous species on hot substrates. SiH4 (g) → Si(s)+ 2H2 (g) at 650 °C Ni(CO)4 (g) → Ni(s)+ 4 CO(g) at 180 °C Also can deposit, Al, Ti, Pb, Mo, Fe, B, Zr, C, Si, Ge, SiO2, Al2O3, MnO2, BN, Si3N4 | Oxidation Using oxygen gas to produce oxides. SiH4 (g) + O2 → SiO2 (s)+ 2H2 (g) at 450 °C 2AlCl3 (g) + 3H2 + 3CO2 → Al2O3 (s)+3CO (g)+ 6HCl(g) Also can deposit TiO2, Ta2O5, SnO2, ZnO |
| Reduction Use hydrogen gas to reduce halides, carbonyl halides and oxyhalides SiCl4 (g)+ 2 H2 → Si(s)+ 4HCl (g) WF6 (g)+ 3 H2 → W (s)+ 6HF (g) Also can deposit, Al2O3 , TiO2, Ta2O5, ZnO | Compound Formation SiCl4 (g )+ CH4→ SiC(s)+ 4HCl(g ) BF3 (g )+NH3→ BN (s)+ 4HF (g ) TiCl4 + ½ N2 + 2H2 = TiN (solid) + 4HCl (gas) A variety of carbide, nitride and boride films can be formed. |
CVD Process Equipment

1. Gas delivery system – to provide the precursors to the reactor chamber
2. Reactor chamber – Chamber in which deposition and reaction take place
3. Substrate loading mechanism – A system for introducing and removing substrates, mandrels etc.
4. Energy source – Provide the energy/heat that is required to get the precursors to react/decompose.
5. Vacuum system* – A system for removal of all other gaseous species other than those required for the reaction/deposition. Not all CVD variants need vacuum.
6. Exhaust system – System for removal of volatile by-products from the reaction chamber.
7. Exhaust treatment systems – In some instances, exhaust gases may not be suitable for release into the atmosphere and may require treatment or conversion to safe/harmless compounds.
8. Process control equipment – Gauges, controls etc.. to monitor process parameters such as pressure, temperature and time. Alarms and safety devices would also be included in this category.
CVD Process Variables
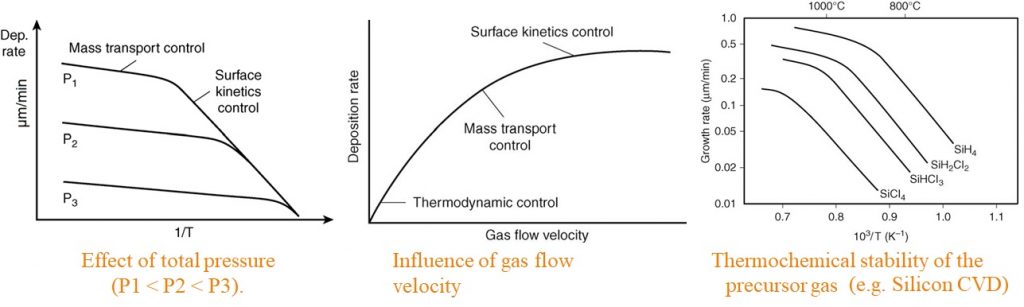
Mass transport control :
It is used to control a process in the vapor in the reactor or from the substrate surface. This occurs frequently at high pressures and high temperatures non-uniform film growth, gas dynamics and reactor design are important
Surface kinetics control :
It exist if the deposition rate is lower than the mass input rate into the reactor. It is favorable for obtaining coatings of uniform thicknesses on substrates with complex shapes.
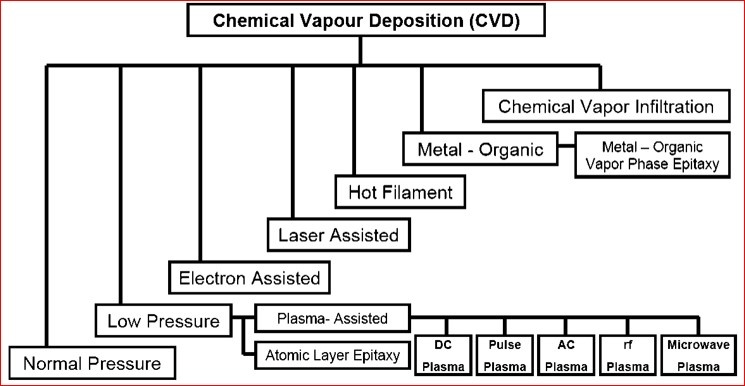
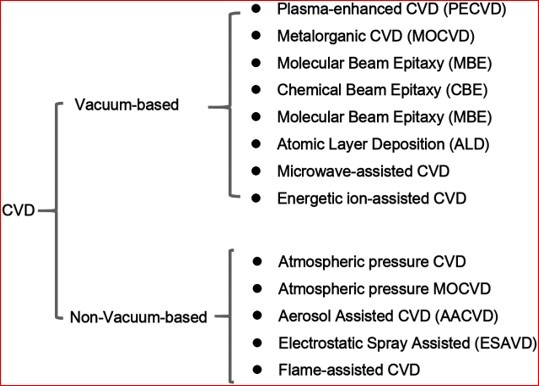
Variants of CVD Process
- Variables are in terms of kinetics of the CVD process. Which subsequently affect the film forming tendency hence many variants of CVD are used for specific film requirements and applications.
- Variants are in terms of Pressure, Temperature, Precursor type, Reactor type, Depositing time, Activated manner
Advantages of CVD
- Producing uniform films with good reproducibility and adhesion at reasonably high deposition rates.
- Non-line of sight process with good throwing power. Can coat complex-shaped components
- It has the ability to control crystal structure, surface morphology by process parameters
- Versatile –any pure element or compound can be deposited. Covers 70% of element table
- ↑ Purity & ↑ Density – nearly 100% of theoretical value achievable.
- Material Formation well below the melting point (SiC of 2700 ˚C can be deposited below 1050 ˚C)
- Economical in production, since many parts can be coated at the same time.
Disadvantages of CVD
- Chemical and safety hazards caused by the use of toxic, corrosive, flammable and/or explosive precursor gases
- The use of more sophisticated reactor and/or vacuum system by CVD variants are not scalable because of high cost, size limitations and narrow applications
Applications of CVD


Coatings Wear, Corrosion, Heat resistance coatings
Solar panels conversion by use of selective absorbers and of thin film solar cells of Si & Ga-arsenide.
Microelectronics & semi-conductors films dielectrics (low and high k), conductors, passivation layers, diffusion barriers, oxidation barriers i.e. IC, Processors(nm), Sensors
Carbon nanotubes, synthetic diamonds & graphene for advance electronic, biological and chemical devices and detectors.
Optical fibers for telecommunication. Optical fibers are produced by coating the inside of a fused silica tube with oxides of silicon, germanium, boron, etc., to get the correct refractive index profile
Want Readymade PPT with perfect formatting? you can buy it for 100INR or 1.5USD, Contact us
References
- “Chemical Vapor Deposition”, Surface Engineering Series, Volume 2, ASM International ®
- Hugh O. Pierson, “Handbook Of Chemical Vapor Deposition(CVD)”, Noyes Publications
- Kwang Leong Choy, “Chemical Vapour Deposition (CVD) : Advances, Technology, and Applications” CRC Press, Taylor & Francis Group 2019
- Jan-Otto Carlsson and Peter M. Martin, “Handbook of Deposition Technologies for Films and Coatings” Chapter -7, Elsevier 2009
- “Chemical vapour deposition – a review”, Nature reviews methods primers, 2021
- Training Handbook “Metal Cutting Technology”, SANDVIK Coromant
