1. Introduction to Selective Leaching
Selective leaching or de-alloying, sometimes is also called as partitioning. Terms such as dezincification, graphitic corrosion, dealuminification, denickelificaton, and the like indicate the loss of one constituent of the alloy, but the general term dealloying has gained wider use in recent years. It is concerned with alloys, not with pure metals. In which one constituent of an alloy is preferentially removed, leaving behind an altered (weak) residual structure. In simple words, it is the removal of one element from a solid alloy by corrosion process.[1,2]
Dealloying or selective leaching corrosion is a form of corrosion is which can be defined as a process where one or more components in a solid solution are either replaced or lost through electrochemical interactions. The potential difference between the alloying elements is the driving force for the preferential attack on the more “active” element in the alloy.
Overall dimensions of original material tend to be retained, residual material is spongy and porous and often brittle in nature. It occurs mostly in solid solution alloys which are homogeneously dissolved at atomic level. The active metal is removed from the alloy by a microscopic-scale galvanic corrosion mechanism in specific environment. The most susceptible alloys are the ones containing metals with high distance between each other in the galvanic series, e.g. Cu-Zn in brass. The elements most typically undergoing selective removal are Zn, Al, Fe, Co, Cr, Ni etc.
1.1 History
During World War I the dezincification of brass condenser tubes was a major problem that kept more British ships out of action than the efforts of the German navy. This problem lessened with the development of more corrosion-resistant alloys, and dealloying is no longer a major problem for most marine operators.[4]
1.2 Comparison with galvanic corrosion
| Galvanic corrosion | Selective leaching | |
| Driving force | Potential difference | Potential difference |
| Location | Between two metals | Within the alloy |
| Coating as a method of prevention | Coating on the surface of the metal can be applied for protection | Coating on the outside surface of the alloy is useless and might enhance corrosion |
| Corrosion rate/ speed | Faster | Slower |
| Detection | Easier | Harder |
| Prevention | Easier | Harder |
| Danger | Less | More |
1.3 Examples
a) Dezincification
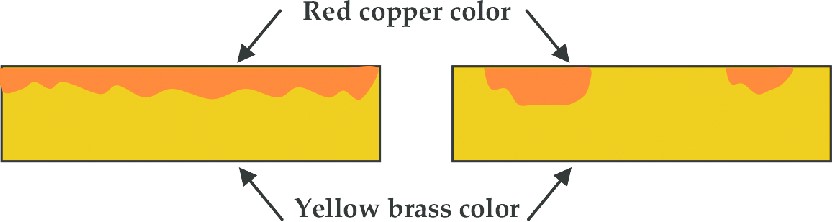
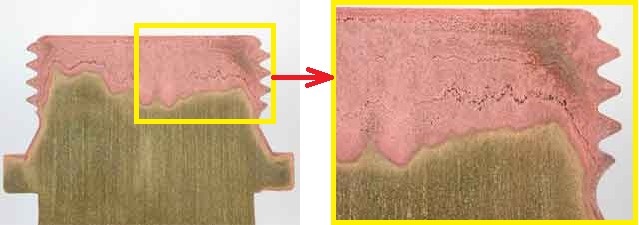
During dezincification, the more active zinc is selectively removed from the brass, leaving behind a weak deposit of the porous and more noble copper-rich metal. dezincification can proceed in the absence of oxygen. Oxygen also enters into the cathodic reaction hence increases the rate of attack when it presents. Example of selective leaching is “Dezincification” in common “yellow” brass 30Zn 70Cu, “dezincifies” to “red copper-rich” structure.
Dezincification can be done either or both of following ways…
| Uniform type | Plug type |
| – Uniformly reduces the wall thickness of the valve or fitting. It occurs relatively uniformly across the material. – Layered dezincification is more common in acidic environments and more common in the alloy if the zinc content is higher. – usually found in high brasses (high[Zn]), acid environments Example[6] : -Penetration of approximately 50% of the pipe wall occurred after several years in potable water service. -Uniform layer type dezincification occurs in tooth of gear wheel. -It also occurs on the inner surface of admiralty brass heat exchanger tubes when exposed to water at pH = 8.0 and temperature range 31-49°C. | – It penetrates deeply into the sidewalls of valves and fittings. – occurs when the alloy is exposed to waters which are either neutral or alkaline and contain a high salt content and the zinc content is low. – usually found in low brasses, alkaline, neutral or slightly acid environments. Example: -It is found particularly in a -brass heat exchanger pipes. If the heat exchanger is not cleaned and dried, differential aeration cells are formed in which the brass dissolves. The corroded region is filled with the re-precipitated copper. |
Generally, Cu-Zn alloys (Brasses) containing>15% Zn are susceptible to dezincification [3]. If Red brass (15% Zn), Naval brass (35% Zn), Muntz metal (45% Zn) is immersed chloride solution at 80°C shows loss in tensile strength 5%, 30%, 100% respectively [2].
b) Graphitic Corrosion
Another popular and most occurring example of selective leaching is in grey cast iron. Cast iron is the cheapest engineering metal (2-4% C, 1-3% Si). Hard, brittle, easily cast; carbon present as microscopic flakes of matrix graphite within microstructure.
In some environments (notably mild, aqueous soils affecting buried pipe) the Fe leaches out slowly and leaves graphite matrix behind appears graphitic, soft which can be cut with a knife. Pores usually filled with rust. Original dimensions are retained, the graphite is cathodic to iron, and an excellent galvanic cell exists. The iron is dissolved, leaving a porous mass consisting of graphite, voids, and rust. The cast iron loses strength and its metallic properties. [1,3].
Graphitic corrosion is happening only in grey cast iron because Nodular and Malleable cast iron don’t have any graphite network and white cast iron have no free carbon to start reaction [2].


Gray cast iron sometimes shows the effect of selective leaching out of iron in mild corrosive environments. The surface layer of the iron becomes like graphite and it can be easily cut with a knife. Because of the attack, the iron or steel matrix is dissolved and an interlocking nobler graphite network is left. The graphite becomes cathodic to iron and a galvanic corrosion cell is formed. Iron is dissolved and a porous mass of voids and complex iron oxides is left behind. This graphitized cast iron loses its strength and other metallic properties (Fig. 3), but to a casual view it looks dirty but unchanged in shape, which can lead to dangerous situations. Graphite corrosion does not occur in nodular and malleable cast iron.
One common mistake in the books is to use the term ‘graphitization’ rather than graphitic corrosion. Graphitization occurs when a low alloy steel is subjected to high temperature for an extended time period. Graphitization results from the decomposition of pearlite into ferrite and carbon, whereas in graphitic corrosion the gray cast iron is selectively attacked. The presence of graphite is necessary for leaching to take place.[6]
c) De-aluminification
Copper alloys containing more than 8% Al may be subjected to the preferential dissolution of aluminum component of the alloy. The α-phase of aluminum-bronze is attacked and a porous residue of copper is left behind.
d) De-nickelification
Although, not common, the de-alloying of nickel in 70-30 Cu-Ni alloy has been observed under low flow conditions.
2. Mechanism
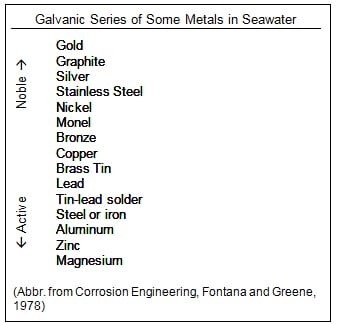
More anodic element in galvanic series (in fig.5) relative with each other, is more susceptible to get leached, for example zinc and copper having larger EMF gap so more anodic Zn is more willing to be leached.
Generally, four mechanisms of de-alloying are proposed [1,9]
a) Dissolution-redeposition
Dealloying has been shown to occur by at least two different mechanisms. the entire alloy dissolves, and noble constituent redeposits on the corroded metal surface (Cu in α-brass). Active constituent gets leached away (Zn in α-brass).
This mechanism occurs in Cu-Zn alloys but may not occur in other alloys like Cu-Au, Au-Ag. For other alloys it may be possible that other mechanism is effective. So, limitation of this model is it doesn’t explain for those alloys where noble metal doesn’t dissolve.
b) Selective dissolution (Volume diffusion)
In other circumstances diffusion removes only the more corrosion susceptible active constituent, leaving an altered noble porous matrix. Both mechanisms have been shown to occur simultaneously on the same metal surface [4]. It should be noted that this diffusion occurs only few layers then further no dealloying occur because diffusivity is low at room temp. Figure 6 shows copper deposits on the surface of dezincified alpha brass. Similar deposits have been reported on cupronickels and Monel.

c) Surface diffusion
De-alloyed surface is rough, porous and loosely packed, so alone atoms have higher surface energy, they try to agglomerate, and often form islands of noble metal clusters, which can be seen as pits, i.e. plug type dezincification. This clustering of atoms creates flush surface of alloy beneath available for further dealloying to continue.
d) Percolation model
It is one kind of limiting factor for de-alloying to occurs, by various researches this model states that there is need to have minimum critical concentration level (threshold level) of constituent for de-alloying to occur.
i.e. in Cu-Zn alloys if Zn < 18% de-alloying doesn’t occur, red brass has 15% Zn so dealloying doesn’t occur, but in yellow brass Zn is more so de-alloying occurs. For the zinc-copper and aluminum-copper systems, the threshold is 18 ± 2 at.% for zinc and 14 ± 2 at.% for aluminum, respectively.
3. Factors affecting selective leaching
- Different metals and alloys have different electrochemical potentials (or corrosion potentials) in the same electrolyte. Modern alloys contain a number of different alloying elements that exhibit different corrosion potentials. The potential difference between the alloying elements is the driving force for the preferential attack on the more “active” element in the alloy. If the ΔE = EN – EA is large then chances of de-alloying are more.
- Higher the content of the active element more is the dealloying. i.e. Cu-18%Zn, Cu-40%Zn. Former is called alpha brasses and later is called as the famous Muntz metal. So, the dealloying will increase in higher concentration of Zn because the ΔE is going to be the same in both cases.[1]
- Increased oxygen level in envoironment may increase corrosion [2].
- Temperature increment may lead to corrosion e.g. Graphitic corrosion [3].
- Service environment also affects, following table shows various alloys that experience de-alloying in respective environments.
| Alloy | Environment | Element removed |
| Brasses | Many waters, especially under stagnant | Zinc (dezincification) conditions |
| Grey iron | Soils, many waters | Iron (graphite corrosion) |
| Aluminium bronzes | Hydrofluoric acid, acids containing chloride | Aluminium (dealuminification) |
| Silicon bronzes | High-temperature steam and acidic species | Silicon (desiliconification) |
| Tin bronzes | Hot brine or steam | Tin (destannification) |
| Copper nickels | High heat flux and low water velocity (in refinery condenser tubes) | Nickel (denickelification) |
| Copper-gold single crystals | Ferric chloride | Copper |
| Monels | Hydrofluoric and other acids | Copper in some acids, and nickel in others |
| Gold alloys with copper or silver | Sulfide solutions, human saliva | Copper, silver |
| High-nickel alloys | Molten salts | Chromium, iron, molybdenum and tungsten |
| Medium-and high-carbon steels | Oxidizing atmospheres, hydrogen at high temperatures | Carbon (decarburization) |
| Iron-chromium alloys | High-temperature oxidizing atmospheres | Chromium, which forms a protective film |
| Nickel-molybdenum alloys | oxygen at high temperature | Molybdenum |
Table 1 Effect of environments in alloys[6]
4. Advantages, Disadvantages
a) Advantages
- Enrichment of silicon observed in the oxide film on stainless steels results in better passivity and resistance to pitting.
- Preparation of Raney nickel catalyst by selectively removing aluminium from Al-Ni alloy by action of caustic. dealloying phenomenon extends to the accelerated corrosion in aluminum alloy 2024-T3 and the development of high-surface-area electrodes and catalysts.[8]
- De-alloying is used to produce nanoporous gold items suited for use in catalysis, electrochemical applications, sensors and actuators [2].
b) Disadvantages
- direct relevance to stress-corrosion cracking[8]
- The dezincified portion is weak, permeable, porous, brittle and possesses little aggregate strength. Hence addition of zinc to copper lowers the corrosion resistance of copper.
- Produces the material having lower mechanical properties due to inhomogeneity of material.[2]
5. Prevention
- Addition of Sn in 70-30 brass improve resisting towards dezincification.
- Use a better alloy like “red” brass, Admiralty Brass (70 Cu, 29 Zn, 1 Sn), arsenical Admiralty (70 Cu, 29 Zn, 1 Sn, 0.04 As).
- Cupronickel (70-90 Cu, 30-10 Ni) can be used in severely corrosive environment [2,4].
- Use copper alloys with copper content above 85%.
- Use brass alloys with tin, arsenic or antimony addition.
- Avoid environments where the solution becomes stagnant and deposits accumulate on the metal surface.[6]
- Use cladding process
For readymade Word file + PPT on selective leaching, and with case study of heat exchanger pipe dealloying, all for 100 INR or 1.5 USD, Contact us.
References
- NPTEL Course by Prof. V. S. Raja, “Aqueous Corrosion and Its Control-lecture-25”, IIT Bombay.
- Mars G. Fontana, “Corrosion Engineering’’, Tata McGraw-Hill
- Dr. Derek H. Lister, “Corrosion for Engineers’’, CANDU
- “Uhlig’s Corrosion Handbook”, Third Edition, 2011 John Wiley & Sons, Inc.
- https://www.materials.co.uk/dezincification.htm
- Zaki Ahmad, “Principles of Corrosion Engineering and Corrosion Control”, Elsevier Science & Technology Books
- https://testlabs.ca/public-sector/severe-graphitic-corrosion-in-cast-iron-drain-lines-of-a-health-care-facility/
- ASM Handbook Volume 13A: “Corrosion: Fundamentals, Testing, and Protection”
